
Remain entirely in their elastic state and they require temperature to enable themĪbsolute zero temperature gas has zero energy so molecule restrict their motion Said that this law is not applicable at low temperature:

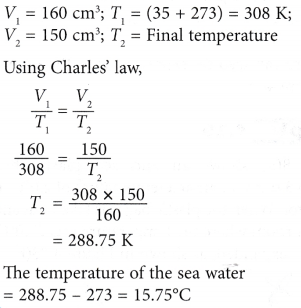
To Charles’s law gas will descend to zero at certain temperature. This is how we recognized that -273.16 o C must represent the coldest temperature. But for real gas the zero volume is impossible which shows that this temperature cannot be attained for a real gas. It is apparent that this temperature of -273.16 oC will be attained when the volume becomes zero. All these straight lines when extrapolated meet at a single point of -273.16 oC (0 Kelvin). The reason is that greater the number of moles greater the volume occupied. Greater the mass of gas taken, greater will be the slope of straight line. For example, at 238 K (10 O C) the volume is 566cm 3 while at 373 K (100 oC) the volume is 746 cm 3. Actually, all the gases are converted into liquids above this temperature.Ĭharles‘s law is obeyed when the temperature is taken on Kelvin scale. This temperature is the lowest possible temperature which would have been achieved if the substance remains in the gaseous state. this can be possible only if we extrapolate the graph upto -273.16 oC. If we plot a graph between temperature on x-axis and the volume of one mole of an ideal gas on the y-axis, we get a straight line which cuts the temperature axis at -273.16 o C. So, by decreasing the temperature the volume of the gas has decreased at constant temperature. Pressure has been kept constant so this gas is obeying Calculate the new volume of the gas at low temperature. Hydrogen is cooled from 127 oC to -27 oC by maintaining the The new values of volume and temperature are V 2 and T 2 The gas in the cylinder is heated both volume and the temperature of the gas Volume of the gas is V 1 and its temperature is T 1. Īmount of a gas enclosed in a cylinder fitted with a moveable piston. The rubber expands as more hot gas is pumped in and the exhilarated particles bounce and push on the inside of the surface, pushing it outward. Volume increases in heated gas because volume isn’t bounded as ball expands pressure is also increases. This behaviour is evident in the air pumps that churn out hot air when their piston is periodically pushed and pulled. However, it is also realized that the pressure is increasing with the increase in temperature provided the volume of the container kept constant. So, the more collision results in higher pressure. Gas pressure is proportional to the magnitude of collision and the force. The impact of this force is inconsequential but collectively the collision exerts pressure on the surface of the container. This rapid collision exerts force on the container surface, that force creates pressure. The particles continuously collide with the walls of the container. James Clerk Maxwell claimed that a space is occupied by a gas is dependent upon the motion of its particles. The temperatures are measured in Kelvin, which is SI unit of temperature. The law determines the linear relationship between volume and temperature. Temperature remains constant for same amount of gas at same pressure. Temperature when the pressure is kept constant.Ĭhanged from T 1 to T 2 and volume changes from V 1 The volume of the given mass of a gas is directly proportional to the absolute
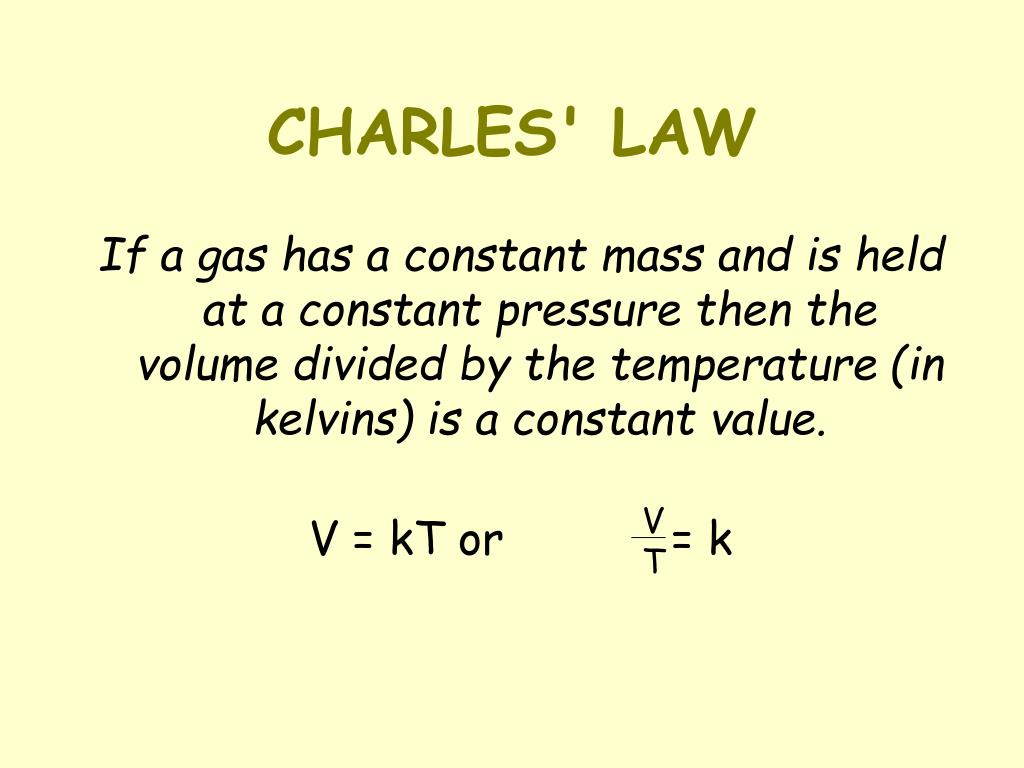
It is a quantitative relationship between temperature and volume of a gas.


 0 kommentar(er)
0 kommentar(er)
